Introduction
This thesis has a broad focus on the long-term post-harvest field storage of the sugar beet crop. It is a compilation of four research papers. These papers collectively explore: how agronomic inputs can impact mechanical properties and storability of sugar beet roots; the use of a handheld penetrometer to assess sugar beet root mechanical properties; how forced ventilation affects quality of, and movement of water in, a bulk of sugar beet roots; and how numerical modelling can be used to understand the airflow and temperature in sugar beet post-harvest storage systems. The research project was conducted within the context of the clamp system of post-harvest field storage, as employed in Swedish agriculture. This context includes the Swedish natural, market, and research environments. The research project has drawn extensively from knowledge well beyond this context, and the findings are similarly applicable to a wider context. This includes wherever post-harvest storage of sugar beet roots is employed, be it in a clamp or in the sugar beet processing factory based large pile storage system. The research project had a technical focus, but aimed to remain firmly grounded in the physiology and agronomy of the sugar beet crop. The background to the research project is given in Section 1. It first provides definitions for “Long-term post-harvest storage of sugar beet” as found in the title of the thesis (Section 1.1), then reviews the principles of successful post-harvest storage of sugar beet roots (Section 1.2), and finally outlines the context of the research project (Section 1.3). Section 2 presents both the broad, overarching aims of the project as a whole, plus the specific aims of each study within the research project. The methods of research are presented in Section 3. The results and discussion of Section 4 focuses mainly on the broad findings of the research project, with particular attention on the synergies between the individual studies. The main conclusions are given in Section 5, and a summary of some areas of promise for future research are given in Section 6.
Long-term post-harvest field storage of sugar beet
Post-harvest field storage system
Storage systems employed for food crops range from the harvested crop being housed in highly controlled environments, through to the crop being left in-situ post maturity until it is taken to the next stage in the value chain. The choice of storage system is a combination of the per unit volume value of the crop, and the crop’s tendency to degrade in the available environments (Wills et al., 2007a). For sugar beet, three storage systems are in common use. Two of these are post-harvest storage systems, including the smaller clamp system located in-field (Figure 1), and the large pile system located at the processing factory. A clamp is an old technology used for the post-harvest storage of root crops, consisting simply of a bulk of the crop piled on the earth and covered as necessary with a protective material such as straw or soil (Aliou, 1998). A pile consists of a bulk of harvested roots that can be five meters high and 40 meters wide, or larger (Bugbee, 1982; Gaddie & Tolman, 1952; Shaaban, 2020). The third storage system sees the sugar beet crop left in-situ in the field beyond the end of the period of seasonal growth, where it is protected by the soil and plant canopy. In-situ storage can be employed where mild winters are expected, and harvest occurs just prior to delivery. If clamp formation occurs, it is usually only for a short period. In some environments where sugar beet is grown, post-harvest storage is strongly discouraged owing to the unfavourable environment (Orleans & Cotton, 1952).
The research project this thesis describes was focused on the clamp system of post-harvest field storage, but this thesis also has application to the pile post-harvest storage system. A large component of the research referenced in the following discussion on the principles of successful post-harvest storage of sugar beet was conducted in the context of pile storage. A distinction between these two systems is generally not made here, as both are the post-harvest storage of bulks of sugar beet roots. The in-situ storage system is generally ignored.

Long-term storage
There is no single clear definition of “long-term” for sugar beet post-harvest storage given in the literature. As such, a broad definition of long-term is here given as storage with physiological stability, where physiological stability is “a dynamic state of a living organism characterized by the maintenance of one or more physiological parameters within value ranges that vary only slightly in the presence of disruptive elements” (Lebel et al., 2014). For the conditions in which post-harvest storage is employed for the sugar beet crop, long-term storage is taken as that which extends more than two weeks beyond the harvest date. Two weeks is an estimate of the average length of the initial period of wound healing and elevated rates of respiration resulting from the harvest process. It also fits with the definition of greater than 15 days, given in a presentation in 2012 by European leaders in research into sugar beet root storage (Legrand et al., 2012). It should also be noted that the processes occurring during the implied short-term are still of significance to this thesis. In particular, exposure and reaction to extreme weather.
Principles of successful post-harvest storage of sugar beet
An idea can be seen as a guiding principles when it has been tested in many contexts and situations and still proves effective or correct (Patton, 2015). As such, two guiding principles for the successful post-harvest storage of sugar beet roots are here postulated:
- healthy sugar beet roots store better than unhealthy roots, and
- sugar beet roots store better when held within an optimal
environment.
These are principles that have been found for all agricultural and horticultural products (Wills & Golding, 2016; Yahia, 2019). They are also rather broad, requiring further elaboration before being practical.
Successful storage and quality
Successful storage is ultimately defined by the maintenance of quality of the product. Quality standards of the sugar beet crop are defined in relation to processing. This includes primarily a high sucrose concentration and low content of invert sugars (glucose and fructose), polysaccharides of microbial origin (including dextran), and of soluble non-sugars (α-amino nitrogen, sodium, potassium) in the processable sugar beet material. Quality also includes the maintenance of the quantity of processable material in a delivered sample. Non-processable material is anything that is not healthy sugar beet root material. This includes soil and stones, non-root plant material, root material that will wash away, the water in these components, or root material that is known to interrupt the extraction process such as roots that have thawed after being frozen. When this non-processable material is captured in the sample of a delivered load of sugar beet roots, it is referred to as dirt-tare. Finally, resistance to cutting is a quality trait of importance in processing (Dutton & Huijbregts, 2006; Vukov, 1977). The term storability is used in the description of successful storage, with good and poor storability relating to the situation of relatively lower and higher loss of quality during storage, respectively.
Loss of quality
Loss of quality occurs when the sucrose concentration reduces, the nonsucrose component increases, or when processable material is lost. The primary mechanisms for loss of quality are respiration, moulds and rots, freezing and thawing, and mechanical damage. There are numerous interactions between these processes and many factors that can drive them. Relative quality before storage should not be taken as an indicator of relative storability. High sucrose concentration roots do likely store better (Hoffmann et al., 2018), but quality prior to storage does not capture a lot of the key determinates of quality after storage.
Healthy sugar beet roots
What is plant health?
In their exposition of the term “plant health”, Döring et al. (2012) conclude that “there is no single plant health definition that provides satisfying clarity and consistence.” It is a “fuzzy” term that, if it is to remain of value, requires more context in its definition than can be given in a simple dictionary type definition. This notwithstanding, it is useful to here adopt the possibly outdated, definitely circular definition of plant health as the plant being “free from” particular things. For the plant health dimensions discussed below, in the context of this thesis, it is generally the case that less of that dimension will have been shown to result in a higher level of storability.
Respiration rate
The continued respiration of the living harvested sugar beet root is commonly cited as the major source of sucrose loss during post-harvest storage (Bugbee, 1993; Huijbregts et al., 2013; Wyse & Dexter, 1971). As a dimension of plant health, respiration rate is considered. As a biennial root crop harvested at the end of its first year of growth, sugar beet is not considered to ripen and thus it is not expected that respiration rate will vary with timing of harvest (Elliott & Weston, 1993; Scott & Jaggard, 1993). Evidence that this has been tested could not be found. Differences in baseline respiration rate have been observed between varieties during post-harvest storage (Lafta & Fugate, 2009; Stout & Smith, 1950), but these are relatively minor in comparison to the differences observed when there is an interaction with other dimensions of plant health or the storage environment. As such, the discussion on respiration rates during post-harvest storage are interspersed throughout the following discussion on the principles of long-term post-harvest storage of sugar beet roots.
Varieties and gene expression
Variety is a collection of stable traits, assessed primarily phenotypically (Gemet, 2023). Differences between varieties in storage losses during long-term storage are commonly observed for sugar beet, and similar to respiration, variety is a factor in the long-term post-harvest storage of sugar beet roots that interacts with many other factors. It cannot be taken as a dimension of plant health per se, only as a useful indicator of possible health status of a plant under certain conditions. This may soon change. Relatively new work from both North America and Europe has begun to study the genetic foundations of storability of sugar beet. Madritsch et al. (2020) found clear differences in gene up- and down-regulation during post-harvest storage, Karen K. Fugate et al. (2022) has linked this to respiration rates suggesting it is the transport of sucrose from vacuoles that is controlled and which in turn controls rates of respiration, and Gippert et al. (2022) linked storability to the presence of free amino acids and the down-regulation of genes involved in amino acid degradation. While this work is very exciting for the future of breeding for storage of sugar beet roots, variety is still but a (very useful) proxy for health.
Disease: pre-harvest
Reduced sugar beet root health resulting from diseases of the growing sugar beet plant have been found to increase rates of quality loss during storage. A four year study over 47 fields by NBR in Sweden found correlations between post-harvest storage losses and the prevalence of the pathogen Aphanomyces cochliodes (Persson & Olsson, 2009). Campbell and Klotz (2006) compared root suffering from severe Aphanomyces root rot to those suffering from mild infections. They showed that sucrose loss during post-harvest storage for the roots with severe infection was approximately two to three times as large. The same research lab also compared resistant and non-resistant sugar beet grown in fields with the pathogen Beet necrotic yellow vein virus which causes Rhizomania (Campbell et al., 2008). The non-resistant varieties had increased loss of sucrose during post-harvest storage of some 20 percentage point (14 % compared to 34 %), and a near 20 fold increase in accumulated invert sugars. The same lab again has also examined Cercospora leaf spot, caused by the pathogen Cercospora beticola Sacc. (Karen Klotz Fugate et al., 2022). No differences in losses of quality during post-harvest storage were found. Strausbaugh et al. (2011) studied post-harvest storage systems which included roots from sugar beet infested with Rhizoctonia-bacteria complex and found increased rates of sucrose loss in piles with infected roots. A study ongoing during 2021 and 2022 with the Coordination Beet Research International (COBRI) of which NBR is a member, is investigating the impact on storability of three of the main viruses in the virus yellows complex. An infestation of beet yellows virus, beet mild yellowing virus, or beet chlorosis virus appears to lead to reduced quality during post-harvest storage. A 22 % increase in respiration during post-harvest storage at 5 °C is reported for roots from plants with virus yellows in Vukov (1977, Table 179, with reference to Neeb and Grupe (1960), Zucker., 13). The roots of plant affected with Beet necrotic yellow vein virus were found to freeze more readily than healthy roots (Strausbaugh & Eujayl, 2018). The roots affected by virus yellows, aphanomyces, rhizomania, and rhizoctonia, and the root of plants with cercospora leaf spot, all began post-harvest storage with lower quality (Campbell & Klotz, 2006; Campbell et al., 2008; Karen Klotz Fugate et al., 2022; Strausbaugh et al., 2011). A general conclusion on pre-harvest plant health is that a healthier plant will give healthier roots and better storability. A conclusion in Huijbregts et al. (2013) was that more work is needed around the pre-harvest factors driving root health and quality loss during post-harvest storage. This may include more focus on the incidences of pre-harvest diseases and on disease causing agents outside of those of economic importance to plant growth.
Disease: post-harvest
The fungal organisms Botrytis cinerea, Fusarium spp., Penicillium spp. and Phoma betae are generally recognised as the major damage causing pathogens in post-harvest storage of sugar beet (Bugbee, 1975; Bugbee & Cole, 1975; Legrand & Wauters, 2012; Liebe, 2016; Liebe & Varrelmann, 2016). From the work of Liebe and Varrelmann (2016), the conclusion can be draw that the presence of these pathogen is very widespread in the fields of northern Europe were sugar beet is grown, possibly with the exception of Phoma betae. Leuconostoc mesenteroides subsp. dextranicum is a bacteria commonly found to populate damaged cells of sugar beet roots, particularly after roots have been allowed to freeze and thaw. The presence of a post-harvest disease leads to rots. Regions of rotten cells will either be washed away at processing, or will likely have higher concentrations of non-sucrose components. For example, dextran and levan are not present in healthy sugar beet roots, only forming in the presence of rot forming micro-organisms (Harvey & Dutton, 1993), and invert sugars concentrations have repeatedly been shown to increase with rates of mould growth during post-harvest storage (Campbell et al., 2011; Kenter et al., 2006).
The presence of fungal or bacterial organisms does not necessarily mean a root will become unhealthy. Strausbaugh et al. (2011) notes that many of the common bacteria found with sugar beet roots slow the development of Leuconostoc. Ongoing work presented at the 78th Congress of the International Institute for Sugar Beet Research (IIRB) – Molin (2022) – has found that the fungal and bacterial communities in the soil and on the root post-harvest varied between varieties, and this correlated with their storability. The bacteria ASV-649 was associated with good storability.
Mechanical damage
Mechanical damage is the damage to the crop that occurs from physical actions. For the sugar beet crop, this will be damage related to the actions of machinery. Mechanical damage can occur during the growing season from mechanical weed control or the movement of machinery through the field. It seems reasonable to assume that this source of damage will not be of significance to post-harvest storage. No studies relating storability to mechanical damage occurring during the growing season are known. Mechanical damage at harvest and transport is, conversely, omnipresent and of large consequence. All harvest damage has been shown to reduce the storability of sugar beet roots.
Quality is lost from roots that have suffered mechanical damage through various mechanisms. Cells that have suffered physical damage will heal themselves through a sequence of steps that leads to the development of suberin and lignin-like substances (Ibrahim et al., 2001). This costs energy, which will be drawn from the vacuole stores of sucrose. In damaged cells, the contents including the sucrose stored in cell vacuoles can simply leak away. An open wound is also a relatively easy point of entry to the root for a pathogen. In their study on the infection of Penicillium and Botrytis in storage piles, Mumford and Wyse (1976) suggest that an open wound is “essential for fungus infection”. Finally, if the damage leads to separation of fragments of the root, these may be left in the field. If small fragments do make it to the factory and end up in the test sample, this will likely lead to an increased dirt-tare as they will be washed out of the sample.
Numerous works show higher rates of quality loss during storage as a result of higher rates of damage. Rates of damage are often quantified as rate of exposure to a damage inducing action, such as tumbling in a rotating drum (Kenter et al., 2006). Kenter et al. (2006) found a five to six times greater loss of sucrose during storage from roots exposed to a very high rate of a damage inducing action in comparison to roots harvested under standard conditions. In a commercial setting over 50 days, Ingelsson (2003) found an average loss of sucrose per day of 0.19 % for root that experienced hard harvester cleaning, compared to 0.14 % for a more gentle cleaning. They also reported much higher incidence of moulds post-storage.
Specific types of damage are sometimes quantified, although they are usually found to exist simultaneously. For example, Akeson and Stout (1978) found that with increasing rates of impact, damage types expanded from just bruising, to bruising and surface wounds, and finally to bruising, surface
wounds, plus cracking. Ultimately, the pathway to loss of quality from the categories of physical damage are those discussed above.
Akeson and Stout (1978) showed that even at low fall impacts where no surface wounds or cracks were visible, loss of sucrose and accumulation of invert sugars during post-harvest storage was elevated. This was attributed to damage as bruising. Brown et al. (2002) attribute approximately 12.5 % percent of total sucrose losses from damage to bruising.
Damage to the surface of the root, like bruising, is not as obvious as cracking and may be obscured by soil. A NBR supervised student project (Skyggeson, 2016) found high levels of surface damage increases risk of frost damage. Machine harvested but crack free roots, and hand harvested roots were stored at -3 °C for a short period, then 8 °C for 18 days. It was observed that all the machine harvested roots showed signs of frost damage, while none of the hand-harvested roots displayed frost damage. This was attributed to surface damage.
The most extreme versions of mechanical damage are cracks (from impacts) and slices (from machinery), with the extreme version of cracks and slices being when entire fragments of the roots are detached. Mechanical harvest will result in cracks and slices. The tap root will need to be broken for the root to be lifted and the removal of the top of the root by slicing is a requirement of processors to ensure standards of quality. Acknowledging this, the test standards for harvest assessment from the IIRB take a root tip
break of two centimetres or less, and a topping diameter of five centimetres or less, as the zero-loss reference levels (Schulze Lammers et al., 2015). Akeson et al. (1974) showed that topping induces high rates of respiration from wound healing, and high rates of moulds and rots later in the post-harvest storage period. This ultimately lead to higher rates of sucrose loss, with 12.6 % lower total sucrose after post-harvest storage in topped roots compared to non-topped roots.
Mechanical harvest and handling in general provides numerous opportunities for mechanical damage: defoliating, topping, lifting, cleaning, transport within the machinery, and transfer between intermediary steps. Olsson (2008) found that 80 – 90 % of mechanical damage occurs in the harvester. The use of force sensors through a harvester showed repeated force applied on the roots of up to 75 impacts over a 12 second period (Tordeur, 2018). The largest transfer of energy has been found to be when there is a large fall, be it into the hopper tank on the harvester, at transfer to a chaser bin, or unloading into a clamp (Steven Aldis, (BBRO, England) 2018-07-11, personal communications). Post-storage, it is commonly observed that loading into transport to the factory is a point at which much kinetic energy is transferred to noise energy when roots land in the trailer: that is, there are large impacts.
Frost damage
Frost damage occurs when the sugar beet root freezes and then thaws again. Freezing causes cell wall damaged from both the expansion of water during solidification and from the formation of sharp crystals. Sugar beet roots freeze at approximately -3 °C (Huijbregts et al., 2013). Frost damage can very quickly lead to complete loss of processing quality owing to leakage or excessive accumulation of dextran in the damaged cells from bacterial activity. Frost damage can occur both while the crop sits in-situ pre-harvest, and post-harvest. The exact tolerance of the in-situ crop will depend on the depth of cold, the length of the cold, and the protection the root is given from the plant canopy (Milford et al., 2002). The average individual sugar beet root left in-situ is more susceptible to frost damage than the average harvested root stored in bulk (Milford et al., 2002; Olsson, 2009).
Mechanical properties
Mechanical properties are a dimension of plant health similar to strength or balance in human health. It relates to the physical robustness and the ability of the plant to be functional in its physical environment. Mechanical properties are quantified in a multitude of ways and in reference to how the plant structure reacts to physical force. Many mechanical properties relate to the strength of a root, but properties on the way a root deforms under stress have also been noted as relevant (Vukov, 1977). A healthy sugar beet root is one with adequate mechanical properties. Too little of a mechanical property will mean a root will not tolerate mechanical harvest and handling. Given the need to slice roots at processing, it is also not desirable to have too much of certain properties. The current status of the commercially available sugar beet genetic material is such that there is little concern for there being too much of certain properties.
A recently completed doctoral research project has studied mechanical properties of sugar beet roots in detail (Kleuker, 2022). That project shares Paper I of this thesis. That thesis focused on the mechanical properties assessed and method developed in Kleuker and Hoffmann (2019). This includes the resistance to puncture forces at the outer five millimetres of a root, and to compression forces in the core of the root. Clear links between higher values for the mechanical properties and reduced loss of quality during post-harvest storage were found (Hoffmann et al., 2022; Kleuker & Hoffmann, 2020, 2021, 2022). The causal mechanism was consistently identified as reduced mechanical damage and thus reduced need for wound healing and reduced mould establishment. The strongest correlations with mechanical properties were with variety. Prior to Kleuker (2022) and its standardised method, similar results were found in Gorzelany and Puchalski (2000, 2003); Nedomová et al. (2017).
In reference to the post-storage process of slicing at the factory, Vukov (1977) notes that other mechanical properties should be considered. Resistance to cutting is a property that is ideally measured directly, although states that it is comparable to a measure that in its description seems similar to puncture resistance as defined in Kleuker and Hoffmann (2019). This is an example of a non-monotonic dimension of plant health, where roots are graded on their resistance to cutting on the scale Soft – Normal – Suberized – Woody – Extremely woody. Vukov (1977) also cites the modulus of elasticity as an important descriptor of how well roots will slice. It is noted that elastic behaviour will also have a bearing on bulk density and porosity of a bulk of sugar beet roots. It has further been suggested that more elastic fruit should suffer less damage than less elastic fruit from a given impact (Ruiz-Altisent, 1991).
While not a mechanical property per se, cell wall content is another property of sugar beet roots that has been linked to storability. Alcohol insoluble residue (AIR) content is a measure of the cell wall content of the root (van Soest et al., 1991) and shows strong correlation with variety and mechanical properties (Kleuker & Hoffmann, 2022). Similarly, marc content is an indication of the post processing pulp content of the root and had been linked to variety and storability (Hoffmann et al., 2018; Vukov, 1977).
Growing season water stress
In a study by Gaskill (1950), it was found that sugar beet roots harvested after growing under moderate drought stress had higher incidences of rot post-storage. After a very long post-harvest storage period of 139 days at approximately 7 °C, the percentage of rots in the drought stressed roots (28.8 %) was double that of roots from sugar beet irrigated until harvest (14.4 %). A follow-up experiment the next year with a shorter two months of post-harvest storage at 18 °C found 9.54 % and 7.85 % losses for the drought stressed and irrigated roots respectively. Kenter and Hoffmann (2008) similarly found increased rates of quality loss from drought stressed roots during post-harvest storage. Higher rates of increase in the concentration of amino N, betaine, total soluble N and invert sugar were observed in the drought stressed roots.
Nutrient deficiencies
A number of nutrient deficiencies have been stated to lead to higher rates of respiration during post-harvest storage. At 5 °C, increased rates of respiration were found from deficiencies of nitrogen (3 % increase in respiration rate), potash (133 %), magnesium (34 %), manganese (12 %) and boron (58 %) (Vukov, 1977, Table 179, with reference to Neeb and Grupe (1960), Zucker., 13). Corresponding increases in the rate of sucrose loss during storage could be expected. The extent of the deficiencies is not known.
An optimal storage environment
Temperature
Temperature is often cited as the most important factor in post-harvest storage (Wills & Golding, 2016). Huijbregts et al. (2013) give the optimum temperature range for the post-harvest storage of non-frozen sugar beet roots under commercial conditions as 2 to 8 °C. The more restricted range of 4 to 6 °C is given in both Bugbee (1993) and English (2020). This minimises the loss of quality per unit time. The temperature should preferably be stable (Wyse, 1978). Within this optimal temperature range, the processes that cause the loss of sucrose stored at the cellular levelled are slowed, as too the rate of loss of processable material. The loss of sucrose is reduced owing to lower respiration (Dilley et al., 1970). Respiration is the primary source of harvested sugar beet roots generally have an early peak at approximately day 3 or 30 °Cd, reach a minimum at approximately 200 °Cd, and then show gradual increase (Akeson et al., 1974; Dilley et al., 1970; Huijbregts, 2009). The early peak has been stated as 2 to 10 times the minimum rate and abates approximately as quickly as it increases (Akeson et al., 1974).
At lower temperatures there will be lower rates of pathogen growth (Sviridov & Kolomiets (2012) quoted in Korobova et al., 2022) and even the avoidance of the growth of some common pathogens (Legrand & Wauters, 2012). Rates of moulds and rots are of increased importance when post-harvest storage extends beyond approximately 250 to 300 °Cd. A large study in six European countries found rates of loss during post-harvest storage correlated most strongly with rates of moulds and rots when storage extended beyond 339 °Cd and out to 996 °Cd (van Swaaij & Huijbregts, 2010). By setting a floor of 4 °C, the accumulation of invert sugars and the trisaccharide raffinose will be less than if it was a lower temperature (Haagenson et al., 2008; Wyse & Dexter, 1971).
Outside of this optimum, temperatures below -2 °C should be avoided (Milford et al., 2002; Wyse, 1978). By avoiding sub-zero temperatures, the severe and acute loss that results when cells freeze and thaw is also avoided. A maximum temperature limit is not known as post-harvest storage is not commonly practiced in climates with ambient temperatures persistently above 15 °C, but it is clear that temperatures above 25 °C should be avoided (Orleans & Cotton, 1952). Given respiration in plants is temperature dependent (Klotz et al., 2008; Vallarino & Osorio, 2019) and seems to increase exponentially in sugar beet roots for the range of temperatures they are commonly stored in post-harvest (Vukov, 1977), there exists the potential for a vicious cycle of temperature and respiration rate to develop in bulks of sugar beet roots. This can rapidly lead to large rates of quality loss.
A generally applicable relationship between temperature and rates of loss of sucrose in stored sugar beet roots under commercial conditions is that loss is 0.02 %/°Cd (Jaggard et al., 1997). Percent is of the total available sucrose at the time of entering post-harvest storage, and the temperature is taken as the ambient temperature. The experimental data that gave these results went out to approximately 700 °Cd. An alternative form of this relationship comes from Legrand and Wauters (2012). They give a rate of loss of 0.013 %/°Cd out to 270 °Cd, and 0.042 %/°Cd between 270 °Cd and 450 °Cd. This gives an average of 0.024 %/°Cd out to 450 °Cd. The increased rate in the second loss of stored sucrose in the early stages of post-harvest storage (out to ca. 300 degree-days (°Cd) – measured in ambient air). Respiration rates in period is again attributed to the increased rates of moulds and rots. Given the aforementioned early peak in rates of respiration, these relationships are only accurate when applied over a long-term post-harvest storage period.
The interaction between temperature and these many other factors suggest it could itself forma principle: that sugar beet roots store better when held within an optimal temperature range. There are, however, two important footnotes that should be appended. The first footnote is that during the initial post-harvest period in which the roots need to heal from the harvest process, the optimal temperature is most likely well above the 4-6 °C range. Fugate et al. (2016) showed that wound healing is more complete in roots stored at 12 °C compared to 6 °C. Only the respiration and transpiration rate of the wounded root stored at 12 °C returned to levels similar to the wound free controls. The loss per degree-day out to the end of the comparable data at ca. 170 °Cd was similar at both temperatures, but it could be expected that the roots initially kept at the higher temperature will store better thereafter. The second footnote is that the absolute best temperature to store roots at to maintain quality over time is that at which the root freeze and remain frozen until processing. An average minimum daily temperature below -9 °C has been suggested as necessary (Bugbee, 1993). This is not a practical solution in the Swedish context, but is achievable in some parts of North America (Backer et al., 1979; Bichel, 1988) and Russia.
The details of the role of temperature in the long-term storage of sugar beet roots is discussed in detail in the literature study written as part of this research project: English (2020).
Moisture
The research into air moisture levels in sugar beet root storage is less extensive than that into air temperature. This is likely due to the situation in naturally ventilated post-harvest storage where air relative humidity levels have been found to be consistently very high and losses from dehydration very low (Huijbregts et al., 2013; Zavrazhnov et al., 2021). Dehydration is the movement of water from the sugar beet root into the surrounding air. It is driven by a differential in the water vapour pressure between the root and the air, and the resistance of the skin of the root to this movement (Carta, 2021b). As relative humidity tends to 100 %, the water vapour pressure differential and thus dehydration tends to zero. For any given relative humidity, the water vapour pressure differential will increase with temperature. The research that has looked at dehydration resulting from low relative humidity originates mainly from North America, where post-harvest storage is longer and active ventilation is used to control temperature in the large pile storage system. It has been shown that lower relative humidity does lead to higher rates of weight loss of roots, but that the effect of temperature when within the ideal range for sugar beet storage (2 – 8 °C) is of little consequence for dehydration (Andales et al., 1980). Over a 15 week storage period in climate chambers at 3.3 to 8.9 °C, mean weight loss at 80-85 %RH was 31 %, while it was only 15 % at 95-100 %RH. Higher rates of electrolyte leakage and respiration rates have also been found in roots stored at lower relative humidity levels (Lafta & Fugate, 2009). Raffinose concentration can increase under mild dehydration, while severe dehydration was found to result in decreased concentrations of many non-sucrose carbohydrates with the exclusion of invert sugars (no change) (Lafta et al., 2020). Pathogen growth does not seem to be increased on dehydrated beets (Bugbee & Cole, 1979). Despite the importance of this transfer of water from sugar beet roots under post-harvest storage, there are no values known to be reported in the literature for rates of transfer per unit root surface area and time (mass flux). Further, it is not well known what the total expected weight loss from dehydration during commercial post-harvest storage is.
Independent of the relative humidity of air, there are also a collection of work that focuses on levels of moisture on the surface of the stored root. It has been noticed that rainfall events have large impacts on the measured dirttare and thus payment received. In a NBR supervised student project, Mårtensson (2017) compared the dirt-tare of samples split at harvest and either analysed directly or stored in boxes for between 15 and 50 days. The dirt-tare of the stored samples was stable at 10.15 % ± 0.85%. The dirt-tare of the samples analysed at harvest ranged from 9.6% to 19.6%, clearly increasing with the closeness of the harvest date to rainfall events. The conclusion was that the dehydration of the soil attached to the root is an important quality driver. At the national level, a correlation between increasing soil water at harvest and increasing average dirt-tare is a regularly observed phenomena (Ekelöf, 2017b).
The movement of moisture can also play an important role in the thermodynamics of the storage system. Cannon (1950) notes the cooling potential of evaporation, suggesting that at temperatures below 21 °C it could remove all the heat of respiration. Zavrazhnov et al. (2021) models the heat exchange at the surface of a 6.5 m high unventilated pile during November in the Kursk region of Russia. They estimate that evaporation at the beet surface accounts for approximately 20 % of the total heat exchange.
Airflow
Airflow is an important factor in the consideration of temperature and moisture in sugar beet root storage. Rates of both heat and moisture transfer between the sugar beet roots and the air will be driven by the differential in these factors between the two phases, and the resistance of the surface to this transfer. The temperature and humidity of the air inside of the bulk of roots will depend greatly on how much of the ambient air is permitted to flow through the bulk, which in-turn will impact the differential. The resistance of the surface to transfer is proportional to the speed of airflow. There is one known study that directly measures airflow in a bulk of sugar beet roots. Tabil, Kienholz, et al. (2003) measured under controlled conditions the velocity-pressure relationship of air forced through bulks of sugar beet roots with various sizes and foreign material percentages. This has resulted in a series of data on the permeability of the bulk that can be applied in engineering studies of the fluid dynamics in sugar beet post-harvest storage. The lack of studies that directly measure airflow in sugar beet root stores is likely contributed to both the difficulty of the working environment and the more insidious nature of low and variable airflow in comparison to factors like temperature. Airflow rates in the ventilated pile systems are quoted to be between 10 and 20 cubic feet per minute (cfm) per ton of roots (Backer et al., 1979; Downie, 1950), which is equivalent to approximately 0.018 to 0.037 m3/h/t.
Oxygen and Carbon
The impact of the levels of gaseous oxygen and carbon in post-harvest storage are generally discussed under the rubric of controlled- or modifiedatmosphere storage. Controlled atmosphere refers to the situation where the levels of gas concentrations, temperature and humidity are regulated to the point of control (e.g. constant levels are maintained), while modified atmosphere refers to a lower level of regulation such that the atmosphere is regulated in a certain direction (e.g. accumulated gases are trapped within the storage system). Wyse (1973) is the only known study into how controlled atmosphere storage could be applied to the storage of sugar beet roots. It was found that in comparison to normal air, a 5 % concentration of carbon dioxide did not have a large effect on storability, while a 5 % concentration of oxygen with 0 % CO2 reduced sucrose losses from 1.3 % to 0.4 %. It is not known if it is possible to reach and maintain these relatively high and low concentrations, respectively, in an open post-harvest storage environment.
Ethylene
It has been shown that sugar beet roots do react to the concentration of ethylene under storage, but also that the effects are likely of little consequence under commercial conditions (Fugate et al., 2010). When roots were places in controlled atmospheres with the concentrations of 0.020 and 0.11 μL/L, an initial average increase in respiration of 55 % was observed through to 48 hours. This effect had disappeared by 72 hours. At the same time, in a large commercial non-ventilated pile, a maximum concentration of 0.028 μL/L was found after 67 days. Levels were only 0.0057 μL/L at 30 days.
Other treatments
Other post-harvest treatments have been examined in sugar beet root storage. Finger et al. (2021) studied how the volatile organic compound methyl jasmonate effects dehydrated roots, finding a positive but small impact. Iztayev et al. (2021) studied how a high concentration of ozone effects the development of moulds and yeasts in covered piles, finding reductions primarily in yeast development. The ozone concentrations used were toxic to humans, raising safety concerns. The application of lime on stored roots at rates of ca. 1 % w/w has been shown to reduce storage losses, presumably by reducing mould growth (Huijbregts et al., 2013; Olsson, 2012).
Summary of principles
As a final note on the principles of post-harvest storage of sugar beet roots, it should be noted that both principles are required to be in place for successful long-term post-harvest storage. Each principle is individually a necessary condition, but not a sufficient condition. When these principles are viewed as existing on a continuum, the higher the plant health and the closer to the optimum environment, the more successful long-term post-harvest storage will be.
Swedish conditions
The use of the clamp system of post-harvest field storage as employed in Sweden is determined by the conditions of the industry agreement, and presumably originates from multiple factors in a chain of historical path dependence. The start date of long-term post-harvest storage in Sweden is based on historical climate data and is defined in the industry agreement. The end date and thus length of long-term post-harvest storage depends on the annual level of production and conditions at the processing factory.
The natural environment conditions: Climate
For the years 2013/14 to 2022/23, daily mean temperature for the sugar beet growing region of Sweden (Figure 2) during the period 1 December to 14 February varied between 0 and 5 °C (Figure 3). During this period, extremes of -15 and 14 °C were recorded. The warmest three day period over this time span was an average mean temperature of 9.2 °C in December 2015, and the coldest three day period was an average mean temperature of -6.8 °C in January 2016. Wind speed averaged 4.5 m/s, originating predominantly from the West (25.0 %) and South West (23.5 %) octants. Mean relative humidity over this period was 91.9 % and average cumulative precipitation 124 mm.

The market conditions: Industry agreement and contracting
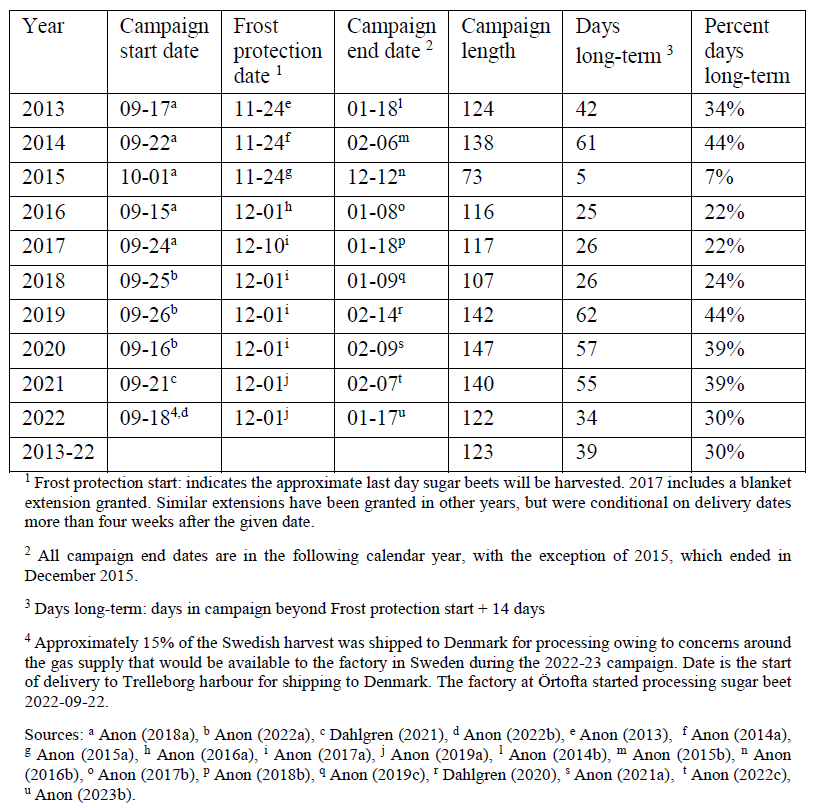
Many of the factors relating to harvest and post-harvest storage of the sugar beet crop in Sweden are specified in the collective agreements negotiated between the grower’s representative organisation and industry. This includes when a cover should be applied and what properties the cover should have, how much a grower is compensated for the use of the required covers, and how the crop will be transported to the processing factory (Anon, 2019a). The risk of in-situ frost damage varies across the sugar beet growing regions of Sweden (Ekelöf, 2017a). In all locations, this risk is deemed too high in the latter months of the processing campaign and a single critical last date for harvest is codified in the industry agreement, as the Frost protection date (Table 1). This date is occasionally adjusted during the processing
campaign as harvest, processing, and post-harvest storage conditions evolve. The varieties of sugar beet grown are those permitted under the agreement between industry and the growers. The information provided on the available varieties was expanded in 2022 to include indication as to which varieties likely store better.
Contracts for delivery are signed with individual growers approximately 12 months prior to the start of a given year’s harvest and processing campaign. Following the deregulation of the sugar beet industry across the European Union in 2017, production is not bound by a mandated maximum volume. Industry does, however, set a maximum volume they will contract. Individual growers register the area and approximate volume they wish to grow and deliver. Should the aggregate total of the individual registrations exceed the industry set maximum volume, the individual contacts offered are adjusted accordingly. Otherwise, contracting is based on the registered interest.
Payment for delivered sugar beet is made primarily on clean root weight at 17 % sucrose concentration equivalent, sucrose concentration, and dirt-tare (Anon, 2019a). Clean root weight is calculated as delivered weight multiplied by (1 – dirt-tare). Dirt-tare is calculated as the percentage of the sample that is washed away during testing, plus a fixed deduction. Beyond the conversion from delivered weight to clean weight, a penalty is deducted for dirt-tare, the grower pays for the transport of the dirt-tare fraction of each delivered load, and loads with dirt-tare of 22 % or higher are rejected for delivery. No material that has been frozen and then thawed should be accepted. The conversion of clean root weight to 17 % sucrose concentration equivalent is achieved by multiplying the weight by the fraction of the
sucrose concentration of the delivered load over the 17 % reference value. A further price adjustment is made for each tenth of a percentage point difference in sucrose concentration from the 17 % reference content. Bonuses are paid on a sliding scale for early delivery – to compensate for lost growth potential – and for late delivery – to address loss of processable material during post-harvest storage. A per tonne payment is made for roots that are stored under an approved cover after a given date. This was 15
November in 2022 (Anon, 2019a).
The payment schedule guides the growers decisions around harvest timing and rates of harvester based cleaning of the roots. In 2022, and considering the entire harvest and post-harvest storage stage of production in which most of the non-washing cleaning of the crop occurs in the harvester, the balance of the bonuses is to encourage the delivery of healthy entire roots. In previous years in which penalties for higher dirt-tare were greater and the value of the mass of root lower, the payment schedule incentivised clean roots (Olsson, 2017).
Post-harvest field storage under Swedish conditions
Sugar beet harvest in Sweden starts in mid-September. Harvest dates on individual farms will be guided by the contracted delivery schedule, but the recommendation is to as best possible harvest at 5-10 °C and when the soil is sufficiently dry (Anon, 2023a). It is very common that the harvester operator is a contractor. The sugar beet roots are harvested mechanically with tractor-drawn or self-propelled harvesters that have hopper (on-board) tanks of approximately 12 tonnes (see for example Edenhall 753 (Anon, 2009)) up to approximately 30 tonne (see for example Ropa Tiger 6S (Anon, 2019b) or Holmer Tera-Dos T5-40 (Anon, 2019d)). Sugar beet stem and leaf is considered dirt-tare, and the recommendation is for defoliation plus minimal topping to find the balance between damage and regrowth post-harvest (Huijbregts et al., 2013). A similar trade-off between damage and removal of soil occurs in adjusting the cleaning system of the harvester. The harvested roots are then transported through the field either in the harvester or in chaser bins and unloaded into clamps (Figure 1).
In Sweden, harvested sugar beet roots are stored exclusively in clamps. These clamps are up to nine meters wide, and three meters high. The width and thus height of the clamp is limited by the working width of the cleanerloader machinery that loads the roots for transport to processing. Cleaner-loader operators are exclusively contractors, as specified in the industry agreement. The shape this gives the clamps means they are commonly referred to an “A-shaped” clamp (Huijbregts et al., 2013). Elsewhere, this type of clamp may be referred to as a “Maus” clamp in reference to the cleaner-loader used (J. Anderson (Lantic, Canada), personal communications, 2022-11-09). The length of the clamp is determined by the size and shape of the field, but may be many hundreds of meters.
The use of a cover on the clamp during the period of long-term post-harvest storage is required by the industry agreement. The choice of cover type is left to the grower. The cover used was traditionally straw (Olsson, 2013b), but this has largely been replaced with non-woven polypropylene fleece and plastic sheeting applied as necessary for extra frost protection (Olsson, 2007, 2013a). TopTex® is the leading brand of non-woven polypropylene fleece. These covers can be managed with machinery, but some manual labour is usually required (Olsson, 2014; Thorstensson, 2016). The purpose of the cover has always been to modify the post-harvest storage environment towards the optimum. Polypropylene fleece seems to reduce airflow in an open environment substantially. A NBR supervised student project (Skyggeson, 2016) measured a decrease from 5.50 m/s in front of the fleece, down to 0.50 m/s behind it. This has not been tested in the field. The temperature of the clamp does not seem to be greatly affected by polypropylene fleece, although it does seem to provide extra protection in the negative temperatures close to zero (Olsson, 2013a). This extra frost tolerance of a clamp covered with polypropylene fleece is likely a result of drier roots. Polypropylene fleece restricts “sufficient” rainfall from entering A-shaped clamps (Huijbregts et al., 2013). The combination of sufficient water exclusion with sufficient airflow allows the transfer of moisture out of the clamp and into the open air at a rate that generally is not matched by rainfall. This results in the drying of the roots and the soil attached to the roots (Mårtensson, 2017). A cover of plastic sheeting is used to stop all airflow through a section of the clamp. It can be applied to the entire clamp, or from the ground and two to four meters up the sides with the top of the clamp left open to permit excess heat to escape through natural convection. The use of straw as insulation is still commonplace when extreme cold periods are expected.
Beyond the use of covers, communications from industry around clamp construction reflect the scientific knowledge. This includes recommendations that a clamp is constructed with uniform width and height to reduce the risk of frost pockets forming during period of negative temperatures (Huijbregts et al., 2013; Thorstensson, 2016), that low levels of foreign material – i.e. weeds – are present (Anon, 2023a; Olsson, 2013b), and that ideally only entire, healthy roots harvested from healthy stands of sugar beet plants are stored post-harvest (Anon, 2023a, 2023c; Olsson, 2013b). Aphanomyces, rust, mildew, and ramularia are common diseases in the Swedish sugar beet crop, while virus yellows, cercospora, rhizoctonia and rhizomania are less common. Other factors that have been discussed in interactions with growers but which are not in the recurring communication from industry include the clamp size (English, 2022), the size of roots (Huijbregts et al., 2013), the use of wind-breaks (Olsson, 2009), or the bearing of the clamp. There are no requirements on these factors.
The post-harvest storage system currently employed in the Swedish sugar beet industry is functional and largely successful. Unsuccessful post-harvest storage appears to be associated with extreme weather, the length of the post-harvest storage period, and the level of activity in the management of the cover. The most common moulds found in clamps in Sweden are Botrytis cinerea, Fusarium spp., and Penicillium spp. (Olsson, 2008). A major issue with the management of the cover is information on the temperature. An ongoing NBR and Nordic Sugar project with temperature sensors reporting live information is aiming to remove this impediment.
Table 1. Sugar beet processing campaign dates and post-harvest storage lengths for
Sweden, 2013-2022
Long-term storage under Swedish conditions
Given the definition of long-term in Section 1.1.2 and the conditions of the industry agreement, the start of the period of long-term post-harvest field storage is calculated as the Frost protection start date plus two weeks. For the ten years prior to the conclusion of this project, the period of long-term post-harvest field storage of sugar beet roots in Sweden lasted for an average of 39 days, or 30 % of the total processing campaign (Table 1). These numbers increase to 54 days and 42 % if the two weeks is excluded. The earliest start date for long-term post-harvest field storage was 8 December in 2013 to 2015, with the latest campaign end date being 14 February in 2019.
An average of 1.93 million tonne of sugar beet roots where harvested annually in Sweden between 2013 and 2022, giving an average of ca. 615 000 tonne of roots stored long-termannually. At a bulk density of 700 kg/m3, that is approximately 880 000 m3 of roots. At the 2021 1-year fixed price contact price of approximately €30 per tonne (17 % sucrose concentration equivalent), that is €18.5 million worth of roots subjected to long-term post-harvest field storage annually.
The broader conditions
The industrial conditions: Rationalisation and Deregulated
The processing stage in the Swedish sugar beet industry has undergone continual change through industrial expansion and rationalisation. When Svenska Sockerfabriksaktibolaget (SSA) was formed in 1907, there were 27 sugar processing factories in Sweden (Kuuse, 1982). Twenty-one of these sites processed sugar beet, and ten refined raw sugar. When Danisco bought SSA in 1992, five beet processing factories remained. In 2006, the second to last of these factories was closed, leaving Örtofta as the nation’s single sugar beet processing factory. From 2022, it also became the nation’s only refinery. Danisco Sugar including the Örtofta factory, was purchased by the company Nordzucker AG in 2008 (Reuters Staff, 2008). A reduction in growing regions accompanied the reduction in processing factories, but it did not proceed at the same pace. The increased per factory production was covered from both increased capacity, but also from longer processing campaigns (Huijbregts et al., 2013).
As a member of the European Union, Sweden’s sugar production has historically been regulated within the Common Agricultural Policy. The sugar market of the entire European Union (EU) was deregulated 30 September 2017 when the production quota system was removed (European Commission, 2017). The deregulation process was agreed as part of the Common Agricultural Policy reform of 2006 (European Commission, 2006). The final result of this deregulation process has seen the EU price become more linked to the world market, but has been somewhat neutral in terms of production levels (European Union Market Observatory, 2023). Should greater production be sort in the future, it would need in the short-term to be supported by increased long-term post-harvest storage.
The global conditions: Sustainable Development Goal 12.3
Sustainable Development Goal (SDG) Target 12.3 is to “By 2030, halve per capita global food waste at the retail and consumer levels and reduce food losses along production and supply chains, including post-harvest losses” (Anon, 2020). This Target mirrors the definition of food loss and food waste given by the Food and Agriculture Organization of the United Nations, with food loss occurring between production and retail, and food waste occurring in retail and the household (English et al., 2019). This current project sits squarely within SDG12.3, given its focus on food loss. Sweden is committed to the SDGs in legislation (Anon, 2021b).
The research conditions: Applied
This research project was instigated by the combined national sugar beet research organisation of Sweden and Denmark: NBR Nordic Beet Research foundation (NBR). NBR was formed on 14 March 2007 by the sugar beet growers and industry (then named Danisco Sugar) of Sweden and Denmark (Olsson, 2007). NBR was a merger of Sockernäringens BetodlingsUtveckling (SBU) in Sweden and Alstedgaard in Denmark, but its ancestry can be traced back to at least the early 1920s when the research focus of the then SSA expanded from only breeding to include soil analysis and growing technique (Kuuse, 1982). Over this time and at its core, the mandate given to the researchers has remained constant: applied research for the advancement of the national sugar beet growers and industry. This project sits firmly within this applied research tradition.